Click on any of the subcategories below to jump to that section:
- 3 Myths About Strontium
- The 3 Different Types of Strontium Salts You Should Know About
- Myth#1: Strontium Increases Fracture Risk
- Myth#2: Strontium Supplements Have Side Effects
- Myth#3: Strontium Skews DEXA Results Too Much To Be Reliable
- Deciding on Dosage
- When to Take Strontium Citrate
- Strontium Safety Issues
- Key Takeaways From This Article
What is Strontium?
Strontium is a mineral from the earth that is naturally found in your body in small amounts.
In fact, 99% of the total amount of strontium in the body is found in bone, a trait it shares with calcium.
Experts now believe strontium is, “an essential trace mineral necessary for the optimal normal development and accretion of peak bone mass, and the sustained health of our bones.”
Along with bone-building elements calcium and magnesium, strontium is a member of the elements in Group 2 on The Periodic Table – meaning it shares some similar chemical properties. Strontium is found in varying amounts throughout the world in the soil, and thus in plants grown in the soil, and in the water – so it is a natural part of our diet.
In the U.S., Canada and Europe, the typical diet contains 2–4 mg strontium/day. However, commercially-produced plant foods grown on fields using synthetic fertilizers, pesticides and herbicides have far lower levels of strontium than their organic counterparts.
Plus, it is well-known that our consumption of plant foods is far below recommended amounts – so it is unlikely you are getting sufficient strontium in your diet. To learn about the most strontium-rich foods, check out our Strontium Food Sources page.
Consumers of conventionally-grown foods are at risk of strontium deficiency, which, as you will soon see, is a factor that increases our risk of osteopenia/osteoporosis as we age.
However, you may have heard some less-than-confidence inspiring things about strontium. So I’m here to set the record straight…
3 Myths About Strontium
Having reviewed the published research on the different forms of strontium: natural strontium, the strontium ranelate drug, along with the radioactive form of strontium, I can confidently assure you that the natural form of strontium, strontium citrate, is a safe and effective bone health supporter. That goes for both the density and the tensile quality of your bones. (I’ll explain more in detail on how they differ shortly.)
But there are still several misconceptions about strontium citrate pills:
- Strontium Increases Fracture Risk. Some strontium critics conclude that strontium causes the outer cortical bone to become thicker, reducing tensile strength (resistance to breaking under tension) and increasing the risk of fractures. However, peer-reviewed research shows otherwise.
- Strontium Supplements Have Side Effects. Strontium has been painted a villain due to undesirable and serious side effects of one form, the drug, strontium ranelate…
- Strontium Skews DEXA Results Too Much To Be Reliable. Since strontium is denser than calcium, it affects bone improvement readings on a DEXA scan. So, some question whether strontium is really increasing bone density or if improvements are just a false positive result.
Let’s take a closer, research-based look at each of them. You’ll soon see these misleading statements hold no weight.
But before we do that, it’s important to understand that there are different forms of strontium – and they all do different things…
The 3 Different Types of Strontium Salts You Should Know About

Natural Strontium Salts
The element strontium is present in natural strontium salts. The most common stable strontium salts are strontium sulfate and strontium carbonate, which are found in soils, and strontium citrate which is used for nutritional supplements.
Stable strontium itself has never been found to cause any harmful effects — with one caveat: to maintain proper balance between calcium and strontium, more calcium than strontium must be consumed.
In more than 100 years of research on strontium, including a number of recent human studies discussed below, no adverse effects have been reported for any natural stable strontium salt when more calcium than strontium is consumed.
Natural strontium salts are safe (at reasonable dosages, of course) and highly beneficial.
Neither of the two forms discussed next are safe. In fact, each has harmful side effects…

Strontium Ranelate Drug
Strontium’s beneficial effects in the treatment of osteoporosis were reported way back in 1910 — but strontium products never entered the market back then due to confusion between the normal, stable forms of strontium with its radioactive isotopes. What everyone heard about was radioactive strontium for building atomic bombs – and no company was willing to launch healthful strontium products with public perception as it was.
But more recently, the poor track record and serious side effects of the osteoporosis drugs, renewed interest in the bone health benefits of strontium by the French pharmaceutical company, Servier.
Since natural minerals cannot be patented, Servier developed a chemical drug form of strontium called strontium ranelate. Servier now had a patentable, exclusive strontium bone drug which was sold beginning in the early 2000’s under the trade name ProtelosTM.
Ranelic acid is the synthetic compound used to create the unnatural strontium salt, strontium ranelate.
Unfortunately, this drug form of strontium is potentially harmful. For additional sources on its toxicity go to Toxin Profiles and the Agency for Toxic Substances & Disease Registry.
Strontium Ranelate has a long list of adverse side-effects, starting with common ones like:
- Nausea
- Skin irritation
- Fainting
- Loose Stools
- Headache
And less common, but possibly deadly effects like:
- Venous thromboembolism
- Serious autoimmune reactions, such as DRESS (the acronym for drug rash, eosinophilia and systemic symptoms)
Strontium Ranelate and Blood Clots
Overall, the likelihood that you will be among the ones affected may be small– although a review of the research on strontium ranelate published in 2005 states “Strontium [ranelate] caused a 50% increase in the risk of venous thromboembolism (including pulmonary embolism).”
In the health information provided for medical professionals, Servier, the pharmaceutical company with the patent on strontium ranelate states:
“In Phase III studies, the annual incidence of venous thromboembolism (VTE) observed over 5 years was approximately .7%, with a relative risk of 1.4 in strontium ranelate treated patients as compared to placebo.”
In other words, over a 5-year period in the Phase III studies, each year, 7 of every 1,000 participants taking strontium ranelate developed VTE.
Saying that those taking strontium ranelate had a relative risk for VTE of 1.4 compared to placebo means that strontium ranelate increased risk for VTE by 40%.
Natural strontium citrate has never been found to increase risk of VTE.
Strontium Ranelate and DRESS Syndrome
Another potentially deadly side-effect of taking strontium ranelate is DRESS syndrome.
Symptoms of DRESS syndrome typically begin 1-8 weeks after exposure to the medication. Classic symptoms include widespread rash, fever, and involvement of one or more internal organs.
Approximately 50% of patients will have hepatitis (liver inflammation), 30% will have eosinophilia (high levels of white cells in the blood indicating immune system activation), 10% will have nephritis (inflamed kidneys), and 10% will have pneumonitis (inflamed lungs).
DRESS syndrome is often severe and can result in death if not diagnosed early – for which reason, you’re urged to see your doctor immediately if you develop a rash after taking strontium ranelate.
Another important note:
The use of strontium ranelate has never been approved in the U.S. or Canada and is now significantly restricted in Europe, while strontium citrate is freely available.

Radioactive Strontium
Radioactive strontium is formed in nuclear reactors or during the explosion of nuclear weapons.
Therapeutically, radioactive strontium is used to treat metastatic cancer but can damage bone marrow and act as a potential carcinogen itself at high doses.
Radioactive strontium isotopes (85Sr and 89 Sr) are also used as delivery agents for chemotherapy drugs and for diagnostic purposes in kinetic studies. (Kinetics is the study of the rate at which chemical processes occur.) Radioactive strontium is not sold as supplements!
Radioactive strontium, which shows up in imaging and is easily traced, is often substituted for calcium in kinetic research because strontium and calcium behave very much alike in the human body.
All forms of strontium and calcium have strong bone-seeking properties, and both share common transport pathways, so they compete for absorption in the intestines and for reabsorption in the kidneys.
I want to underscore here that this is a competition that calcium always wins, which explains why:
- Strontium cannot displace calcium when adequate supplies of calcium are present.
- Strontium supplements should be taken at a different time of day from calcium, or else little, if any, strontium will be absorbed.
Ok, now that you see the differences in the 3 main types of strontium, let’s dig into the common misconceptions surrounding this valuable mineral.

The Truth About Strontium
Myth #1: Strontium Increases Fracture Risk
Some strontium critics claim that strontium causes cortical bone to become thicker, and since cortical bone is less flexible, may increase risk of fractures.
The scientific facts are so different from these assertions, it’s unclear why they publish such misinformation.
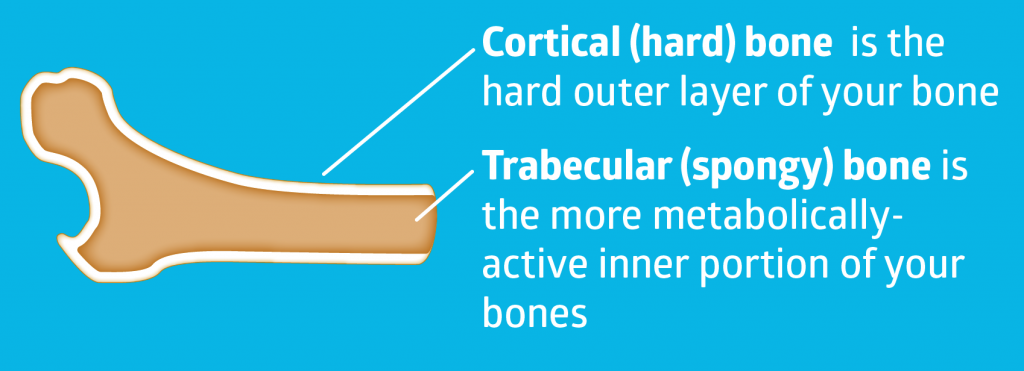
The very tiny amount of strontium ions that deposit in our bones prefer to do so in trabecular, not cortical bone.
Trabecular bone is the soft, spongy, more metabolically-active inner portion of our bones. Cortical bone is their hard, outer layer. If your bones were M&Ms, cortical bone would be the outer candy shell and trabecular bone the soft chocolate center.
Some strontium critics assert that strontium deposits primarily in cortical bone and that somehow translates into reduced bone strength.
They base their claims on these 2 studies:
- Boivin, Deloffre, Perrat, et al.. Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J Bone Miner Res. 1996 Sep;11(9):1302-11.
- Blake and GM, Fogelman I. (2006 JBMR 21(9) 1417-24 Strontium ranelate: a novel treatment for postmenopausal osteoporosis: a review of safety and efficacy. Clin Interv Aging. 2006;1(4):367-75.
But in both cases the authors of the studies drew POSITIVE conclusions about strontium’s bone supporting role, yet the uncredentialed author of the website drew negative conclusions!
Here is the researcher’s conclusion for the first study:
“In conclusion, taken up by bone, Strontium … induced no major modifications of the bone mineral at the crystal level. As a result, a treatment with Strontium should not induce any alteration of bone mineral.”
Nowhere in the above study, is it proposed that strontium is weakening bone.
The second study (Blake and Fogelman) drew this conclusion:
“Strontium … is the only treatment proven to be effective at preventing both vertebral and hip fractures in women aged 80 y and over.”
Again, the study says nothing about strontium weakening bone structure. And neither study shows strontium depositing primarily into cortical bone. It doesn’t. Strontium primarily deposits in trabecular bone.
The new-forming bone in the trabecular part of your bones is far more active and incorporates more strontium ions than the cortical bone. Cortical bone also accumulates far more slowly. So its incorporation of strontium – which is less than 1 strontium ion for every 100 calcium ions even in trabecular bone – is even less than cortical bone.
What the research shows is that strontium, in addition to its numerous beneficial effects that boost osteoblast and slow osteoclast activity, also indirectly inhibits resorption of the calcified bone matrix by stabilizing hydroxyapatite crystals.
Another fact: strontium is the only trace mineral present in human bone whose level in bone correlates with bone compressive strength, i.e., resistance to fracture.
The bottom line is: Strontium citrate improves healthy bone mineralization and resistance to fracture.
Myth #2: Strontium Supplements Have Side Effects
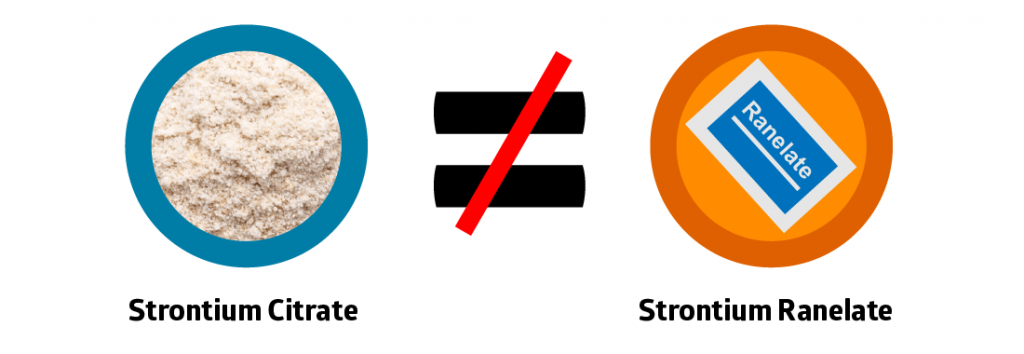
The drug, strontium ranelate, has a long list of side effects, and since the drug has much more media exposure than the health supplement strontium citrate, the two are often confused.
Most blogs, websites, and even doctors do not know that strontium citrate is a completely different molecule from strontium ranelate. Equating them is like suggesting sodium chloride (table salt) and sodium bicarbonate (baking powder) are the same. They both contain sodium, but when you connect that sodium molecule to another molecule, its actions are completely different!
Another issue to consider is that the strontium ranelate drug, Protelos, contains Aspartame – an ingredient which is known for causing reactions on its own.
Natural strontium citrate has never been found to cause any harmful effects– with one caveat: to maintain proper balance between calcium and strontium, more calcium than strontium must be consumed, preferably twice as much calcium.
Myth #3: Strontium Skews DEXA Results Too Much To Be Reliable
The common myth is that strontium supplementation results in larger concentrations of strontium in your bones. And more strontium in your bone causes the DEXA to overstate your bone density increase.
The truth is, strontium does weigh much more than calcium, and its mass does impact DEXA readings…but only a little.

Strontium has nearly twice the atomic weight of calcium.
Because of this difference in mass, strontium’s substitution for calcium weakens X-ray penetration during DEXA scanning and results in overestimation of BMD. But strontium ions replaces less than one calcium ion out of 10, so the overestimation is not great enough to discount DEXA results showing improvements in BMD.
The latest science shows your result may be overstated by 8.5% – 11.2%. But don’t throw the baby out with the bath water. Even after adjusting your score down by about 10%, an increase in bone density is still an increase!
For example, if you gained a modest 1% after taking strontium and you reduce that score by 10%, you STILL GAINED .9% of new bone. That’s a big win when you normally would have been losing bone density!
Don’t be fooled by the strontium critics. The increased density in the strontium studies (including the AlgaeCal studies) are still outright increases in bone density in older adults who should be losing bone. And increased bone density is closely correlated with lowered risk of breaking a bone.
Besides, strontium has been shown to reduce fracture risk independent of bone density. In other words, whether your bone density increases or not, strontium reduces your risk of breaking a bone!
You’ll understand why strontium works as it does when you read the list of strontium’s bone-building effects on both osteoblasts and osteoclasts. (I will be publishing a post on its positive bone-building mechanisms in the near future.)

Deciding on Dosage
How much supplemental strontium do you need for healthy bones?
If you have low bone density, you will receive the most benefit from a 680 milligram per day dose of strontium citrate.
This is the dose that has been used in almost all the human research conducted on stable strontium salts. (It’s also the same dose of strontium citrate provided by 2 capsules of bone-building Strontium Boost.)
If your bones are currently in great shape, you may still wish to consider supplementing with strontium citrate at a dose of 340 milligrams per day. This dose will help protect and maintain the long-term health of your bones.
A personal note: this is my situation. Within a year after I began supplementing with AlgaeCal Plus and Strontium Boost (680 mg strontium citrate daily), my bones were completely healthy. My DEXA results were excellent, showing bones in the healthy, normal range, where they have remained, which I credit in large part to taking AlgaeCal Plus, Strontium Boost and, since it became available, Triple Power Omega 3 Fish Oil.
(You can read about my journey from rapidly advancing osteopenia in my early 40s back to healthy bones)
After two years of excellent DEXA results, I thought I no longer needed extra help and stopped taking Strontium Boost.
But now, after my in-depth review of the current research on strontium – which involved reading more than 70 recently published papers in the peer-reviewed medical literature – I am convinced that strontium citrate has so much to offer that will help ensure my bones remain healthy that I’ve begun supplementing with Strontium Boost again!
Now, however, I’m taking a prophylactic (preventive) dose of 340 milligrams per day, which I also recommended to my husband to help keep his knees healthy, so, at 69 he can keep playing basketball with men half his age.
[ac_banner name=”bbpbiking”]
What is strontium’s optimal dosage?
In the most recently published studies in which the effects of natural strontium salts on bone health were researched, strontium citrate was effectively absorbed and deposited in bone at both a 340 mg per day and a 680 mg per day dose.
It should be noted that:
- The studies showing a 340 mg daily dose of strontium citrate was effective were quite small, including just 10 osteopenic and/or osteoporotic women.
- Participants in a much larger study in which the 680 mg per day dose was used — 172 women who were followed for a period of 7 years — were taking not only strontium citrate (Strontium Boost), but also AlgaeCal Plus. So, all the credit for the highly significant gains in BMD seen every year — which averaged +1.04% per year over the 7-years — cannot be attributed solely to strontium. However, because strontium is the only mineral that both lowers the rate of bone resorption and promotes the rate at which new bone is built, it’s obvious that strontium contributed to the exceptional results seen in this 7-year study.
- It’s also vitally important to recognize that THERE IS NO SINGLE MAGIC BONE-BUILDING BULLET! Building bone requires a nutrient “team effort”! You’ll achieve optimal results when you combine strontium citrate and ALL 12 of the other essential bone-building nutrients. These are: calcium; magnesium; boron; copper; manganese; silicon; nickel; selenium; phosphorus; potassium; vanadium; and zinc. And you’ll need vitamins D3; K2 (in the form of MK-7); and C too. That’s why you’ll be taking all of these bone-essentials when you follow the AlgaeCal protocol (thanks to the Bone Builder Pack)
Avoid consuming calcium for at least 2 hours before taking strontium
As explained above, calcium will be preferentially absorbed over strontium. Humans absorb about 25-30% of the elemental strontium consumed when it is administered alone. But if strontium is taken at the same time of calcium, our absorption of strontium is reduced to just 15-18% if it’s taken with calcium. So it’s important that you take the two minerals at least 2 hours apart.
In additional to calcium-containing foods, foods that contain alginate (found in kelp) lower strontium absorption, and so do certain medications, including quinolone antibiotics, and medications that contain aluminum hydroxide or magnesium hydroxide.
For at least 2 hours before and after taking your strontium citrate supplement, avoid consuming:
- Supplements containing calcium.
- Foods naturally rich in calcium: such as dairy products (cow’s milk, cheese, yogurt, kefir), bone broth, sardines, spinach, kale, collard greens, turnip greens, mustard greens, beet greens, watercress.
- Foods fortified with calcium: such as calcium-fortified almond, rice and soy milks, tofu, calcium-fortified orange juice and other fruit juices.
- Kelp: Alginate, which is found in kelp, reduces strontium absorption so greatly that it is used to prevent strontium toxicity in cases of radioactive strontium poisoning. Alginate is a thickening agent used in sauces, yogurt, puddings and pies.
- Quinolone antibiotics: like calcium, strontium is likely to form complexes with quinolones in the gastrointestinal tract, preventing absorption of the antibiotic. Broad-spectrum antibiotics, the quinolones include ciprofloxacin (Cipro), levofloxacin (Levaquin), ofloxacin (Floxin), moxifloxacin (Avelox), gatifloxacin (Tequin), and others.
- Medications that contain calcium: such as calcium-containing antacids and oral tetracyclines. Calcium-containing antacids include calcium carbonate (Tums & others), dihydroxyaluminum sodium carbonate (Rolaids & others). Tetracyclines include demeclocycline (Declomycin), doxycycline (Vibramycin), minocycline (Minocin), and tetracycline (Achromycin, Sumycin).
- Antacids that contain aluminum hydroxide or magnesium hydroxide: both can reduce absorption of strontium by 20-25%. Antacid medications that contain aluminum or magnesium hydroxide include magaldrate (Riopan), Amphojel, and aluminum hydroxide/magnesium hydroxide combinations (Maalox, Mylanta, others).
- Chelating agents: It is not uncommon for people over age 50 to have accumulated high levels of heavy metals, such as mercury, cadmium and/or lead. If you have been found to have high levels of heavy metals and have been prescribed an oral chelating agent, such as DMSA, take strontium citrate 3-4 hours before or after consuming your chelating medication.
At this point, you’re probably wondering…
When to Take Strontium Citrate
What schedule can help me optimize strontium absorption and let me lead a normal life? I suggest the following:
- AlgaeCal Plus: 2 capsules with breakfast
- AlgaeCal Plus: 2 capsules with lunch
- Strontium Boost: 2 capsules right before bed (on an empty stomach)

Print your own How to Take Your AlgaeCal Supplements PDF.
Why this regimen?
It is best to take strontium supplements and calcium at different times of day to get the most benefit.
Take your strontium before bedtime at night. It has been suggested that bone resorption is most active at night, so taking strontium citrate just before bed may result in strontium exerting more of its antiresorptive effects when they are needed most.
Strontium can be taken with or without food. Just be sure the food does not contain calcium.
Calcium on the other hand, should be taken with food so that when food nutrients are being metabolised, the calcium absorption happens along with the other nutrients in the same digestive phase.
Both the strontium and the calcium supplements are absorbed in our gastrointestinal tracts using the same mechanism. Thus, if we take both the supplements together or at the same time, the two will compete for absorption by the body. Scheduling your supplementation like above, will ensure that this does not happen.
What Else Can Be Done To Optimize Strontium Citrate Absorption?
How to boost your ability to absorb strontium and get the most from your strontium supplement…
Our active intestinal absorption of strontium is vitamin D-dependent and decreases with aging, and with consumption of phosphate additives as well as calcium.
You can further optimize your absorption of strontium by:
Ensuring your intake of vitamin D3 is adequate
What’s “adequate” vitamin D3? Sufficient to bring your blood level of 25(OH)D into the 50-80 ng/mL range. 25(OH)D is the vitamin D form circulating in the bloodstream and the marker typically used to assess vitamin D levels. Ask your doctor to get tested to know your current levels and how much you need to bring your levels up to the healthy range.
Avoiding consumption of processed foods
Phosphate, as well as calcium, significantly lowers strontium absorption. Phosphate additives are used as preservatives in most processed foods, including sodas (regular and diet sodas), breads, rolls, sweet bakery products, tortillas, cereals, savory snacks, crackers, snack/meal bars, pizza, poultry, cured meats, vegetables (processed, not fresh!), egg products (e.g., Egg beaters, powdered eggs), seafood, and candy (including chocolate). Read labels!

Strontium Safety Issues
Individuals with chronic kidney disease
Strontium is eliminated by our kidneys, so it may accumulate in patients with chronic kidney disease, as their kidneys’ ability to filter the blood is compromised.
In patients with chronic renal failure, blood levels of strontium are increased four-fold when creatinine clearance is less than 50 mL/minute. Supplemental strontium should be avoided in individuals with a creatinine clearance less than 50 mL/minute or who are on dialysis. A creatinine clearance rate of 90 or greater indicates normal healthy kidney function. A creatinine clearance rate of 60-89 indicates the initial stage of kidney disease.
Creatinine is a breakdown product of creatine phosphate in muscle; it’s usually produced at a fairly constant rate by the body and is eliminated in urine. How quickly our kidneys filter creatinine from the bloodstream, which is called the creatinine clearance rate, provides a measure of how effectively your kidneys are filtering your blood. (The creatinine clearance rate is also called the glomerular filtration rate because tiny capillaries in the basic structural and functional unit of the kidney, the nephron, are called glomerular capillaries.)
Bone levels of strontium are often high in chronic kidney disease patients on dialysis
People with chronic kidney disease are less able to remove waste and excess water from the blood. Dialysis is a way of carrying out this process. Dialysis fluid can contain high concentrations of strontium, so not surprisingly, some dialysis patients are known to have elevated bone strontium concentrations and a high strontium/calcium ratio in bone. As a result, about 5% of dialysis patients develop osteomalacia (softening of the bones).
However, osteomalacia is also frequently seen in end-stage kidney failure patients who are not yet on dialysis and who have normal bone strontium levels. The reasons for this are that failing kidneys lose their ability to:
- Activate vitamin D (vitamin D is converted into its active, hormonal form in the kidneys –which is the form in which vitamin D helps us absorb calcium from the digestive tract)
- Re-absorb calcium passing through the kidneys in the bloodstream instead of losing it in urine
So, if you are on dialysis or have end stage of kidney failure, ask your doctor for advice on whether to proceed with strontium supplementation.
Key Takeaways From This Article
- Strontium delivers a unique combination of bone health benefits no other nutrient provides. Strontium is the only mineral in our bones that both lowers the rate of bone resorption and promotes the rate at which new bone is built.
- Three types of strontium salts are in use today: (1) Natural stable strontium, e.g., strontium citrate, (2) Unnatural stable pharmaceutical strontium called strontium ranelate, and (3) Radioactive (unstable) strontium.
- Natural strontium salts, (strontium citrate), are safe (at the dosages discussed above and when twice as much calcium is consumed as strontium) and highly beneficial; neither strontium ranelate nor radioactive (unstable) strontium is safe.
- Strontium ranelate comes with risks of unwanted side effects. The long list of adverse side-effects attributed to strontium – ranging from commonly seen adverse effects like nausea and skin irritation to possibly deadly effects like venous thromboembolism and serious autoimmune reactions, such as DRESS – are caused by ranelic acid (the “ranelate” portion of strontium ranelate), not strontium.
- The use of strontium ranelate has never been approved in the U.S. and is now significantly restricted in Europe, while strontium citrate is freely available.
- Don’t worry about strontium taking the place of calcium in your bones. Strontium and calcium share a common carrier system in the intestinal wall, which will always choose to carry calcium rather than strontium from our digestive tract into our bloodstream. Strontium competes with calcium for absorption – and calcium wins every time. Also, strontium is eliminated more readily than calcium from kidneys.
- Strontium is almost twice as large as calcium, and its larger size impacts DEXA readings. Available data indicate that approximately 10% of the increase seen in BMD is due to strontium’s larger size – which also means that 90% of the increase seen in BMD is accurate. Besides, strontium has been shown to reduce fracture risk independent of bone density. In other words, whether your bone density increases or not, strontium reduces your risk of breaking a bone!
- Strontium’s beneficial effects are not due to its replacing calcium in your bones. They are the result of strontium’s bone-building effects on a very wide range of key molecules involved in bone remodeling.
- Strontium increases our ability to absorb and deposit calcium in our bones– yes, calcium. And that’s another reason why the Bone Builder Pack of AlgaeCal Plus and Strontium Boost have not only slowed bone loss, but built new bone for the thousands of people who’ve tried it.
FAQs
What is strontium used for?
Strontium is a mineral that’s used to enhance bone mineral density along with calcium. This mineral increases our ability to absorb and deposit calcium in our bones.
Is strontium harmful to the body?
Natural strontium salts (as opposed to strontium ranelate and radioactive strontium) are not harmful to the human body and can enhance bone mineral density when taken with calcium. On the other hand, Strontium ranelate comes with unwanted risks and side effects and has not been approved for use in the United States.
Are strontium supplements safe?
Natural strontium salts (strontium citrate) are safe when consumed in the correct dose and with the proper amount of calcium. However, strontium ranelate and radioactive (unstable) strontium are not safe due to risks and unwanted side effects
What does strontium do for bone health?
Strontium is the only mineral in our bones that both lowers the rate of bone resorption and increases the rate at which bone is rebuilt.
Which foods contain strontium?
Cereals, grains, and seafood have the highest amounts of strontium, along with Brazil nuts, leafy green vegetables, and dairy products.




moyra earnshaw
January 13, 2012 , 6:37 amThank you for these points but you say nothing about the risk of blood clots.